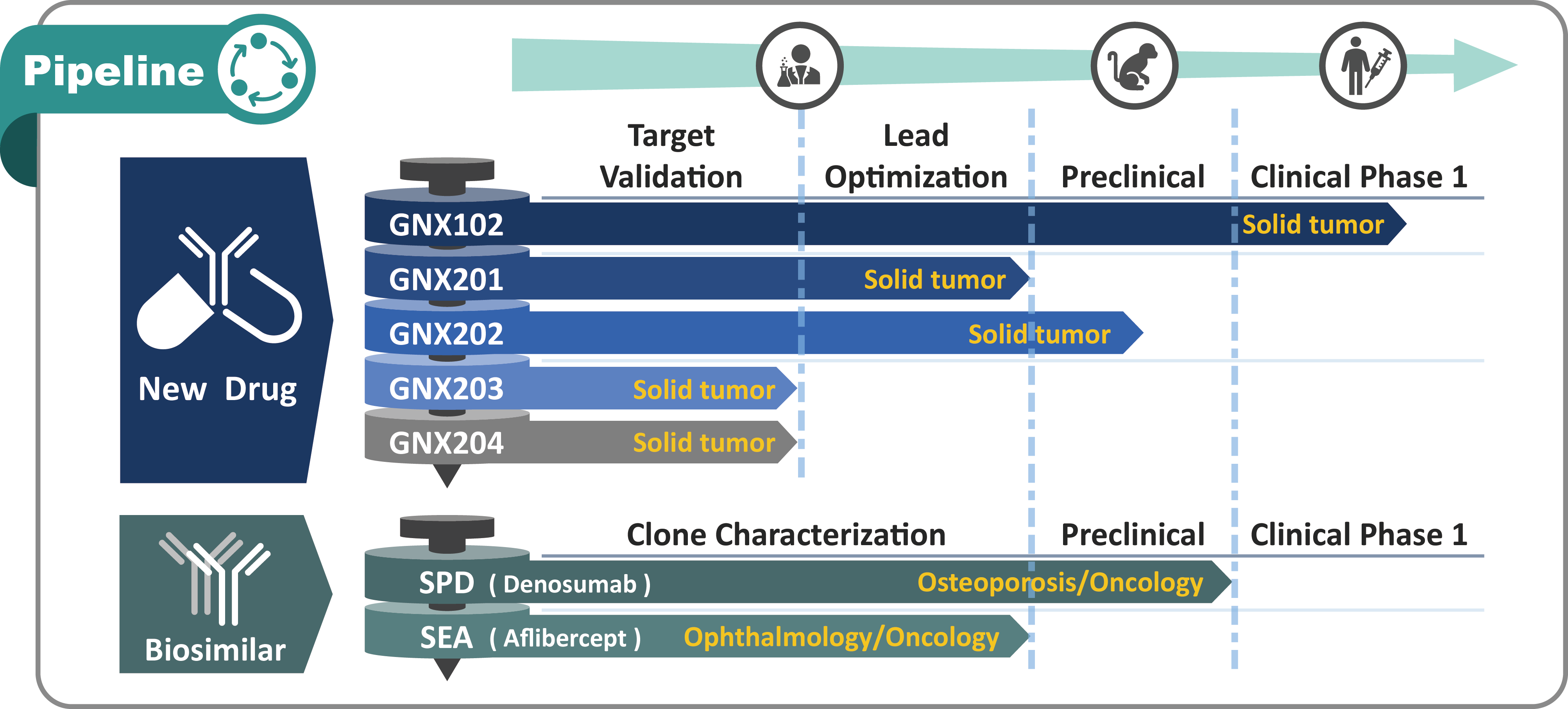
New Drugs Development
“Glycans” are considered to play an important role in tumor metastasis and poor prognosis.Anti-TACAs Antibody: GNX102
GNX102 is a humanized monoclonal antibody developed by GlycoNex Inc.GNX102 is a humanized monoclonal antibody developed by GlycoNex Inc. GNX102 binds novel tumor-associated glycans and exhibited extraordinary tumor suppression in the mouse xenograft models of stomach and colorectal cancers through various mechanisms. GNX102 reacts with a wide spectrum of cancer tissues, showing the therapeutic potential for multiple high-incidence cancer types including colon, stomach, liver, lung, breast, bladder, pancreas, melanoma, uterine, esophagus, prostate, ovarian, and cervical cancers. The combined results of the nonclinical pharmacology, pharmacokinetics and toxicity programs for GNX102 indicate the potential for a favorable benefit to risk profile for use of GNX102 in treatment of solid tumors. IND of GNX102 has been submitted to US FDA in 2019 and the Phase I first-in-human clinical trials are being evaluated and targeted to launch in 2020 bilaterally in US and Taiwan. We look forward to building partnership for GNX102 development.
Novel anti-carbohydrate antibody development
- GNX201
- GNX202
In antibody drugs development, patient safety is a very important issue. Enhance antibody selectivity can help to reduce the side-effects and have a better quality of life. GlycoNex uses an enhanced selectivity technology platform to develop low side-effects and highly anti-tumor efficacy “pro-antibodies”. Pro-antibody can selective activated in the tumor microenvironment to kill tumor cells. Moreover, pro-antibody is more stable in circulation and normal tissue to reduce antibody-dependent side-effects. Pro-antibody can stabilize in circulation and selectively activated in the tumor microenvironment by tumor-associated protease. Therefore, pro-antibody could provide lower side-effects and direct anti-tumor efficacy to patients.
Biosimilar development
Biosimilar is a biologic product highly similar to the approved medicine. GlycoNex has established a comprehensive antibody technology platform for biosimilar development. Since 2010, GlycoNex cooperates with Mitsubishi Gas Chemical Company, Inc. on developing Denosumab and Aflibercept biosimilars. To date, the biosimilar products have demonstrated high similarity to reference medicine and the high-quality antibodies are ready for clinical use.
- SPD
- Denosumab is a humanized IgG2 antibody that inhibits RANK Ligand approved in 2010.
- According to the indications, there are 2 trade names, Prolia for the treatment of osteoporosis; Xgeva for treatment of tumor bone metastases and giant cell tumor of bone.
- GlycoNex cooperates with Mitsubishi Gas Chemical Company and Cultivecs on SPD, biosimilar of Denosumab. SPD have finished cGMP process development in Cultivecs, and ready for clinical trials.
- SEA
- Aflibercept is a vascular endothelial growth factor (VEGF)-binding fusion protein, and invented by Regeneron Pharmaceuticals and approved in 2011.
- According to the indications, there are 2 trade names. Eylea for wet macular degeneration treatment. Zaltrap (ziv-aflibercept) for metastatic colorectal cancer treatment.


