Innovative Drugs
Sweet side of cancer cells as novel therapeutic targetsAnti-TACA mAb: GNX102
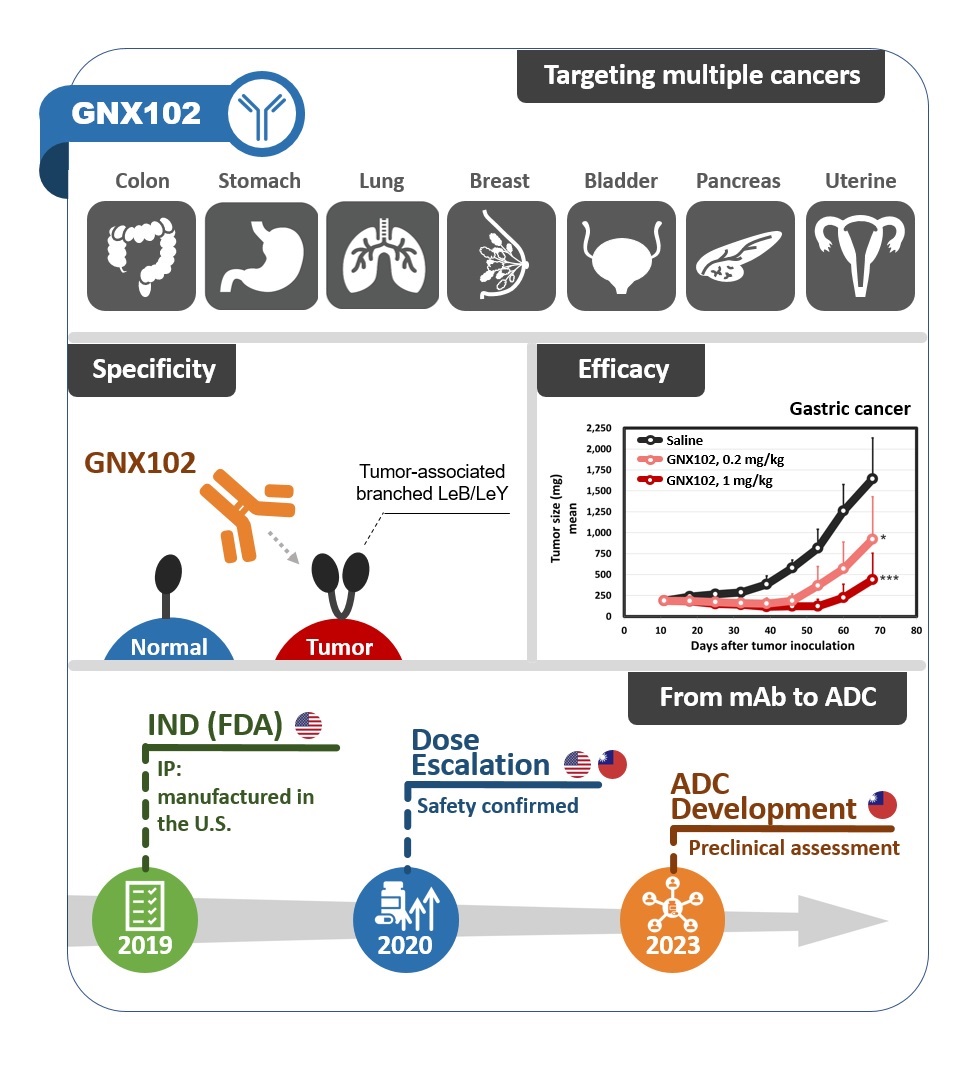
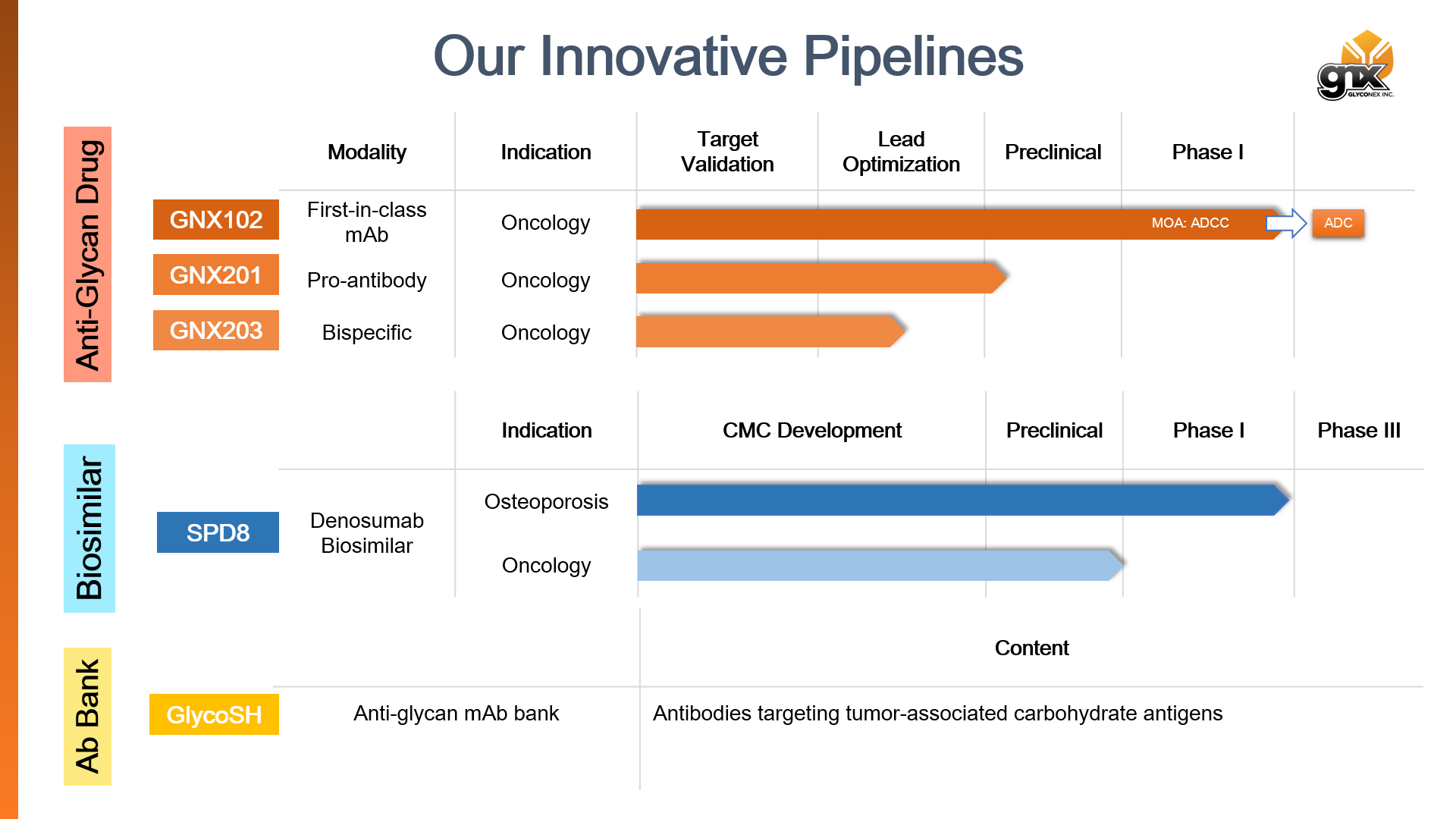
A firs-in-human anti-branched LeB/LeY mAb developed by GlycoNex Inc.GNX102 is a humanized IgG1 antibody developed by GlycoNex Inc. GNX102 binds novel tumor-associated glycans and exhibits remarkable tumor suppression in the xenograft models of stomach and colorectal cancers mainly through Fc-mediated mechanisms such as ADCC. In IHC profiling GNX102 displays reactivity to a wide spectrum of cancer types including colon, stomach, lung, bladder, and pancreas etc, suggesting therapeutic potential of GNX102 for those high-incidence cancers. From the results of nonclinical pharmacology, pharmacokinetics and toxicity programs, GNX102 bring a favorable benefit-to-risk opportunity to treatment of multiple solid tumors. Clinical phase I study of GNX102 was completed in 2023. Safety profile in human has been thoroughly assessed and MTD was determined. ADC program of GNX102 was initiated soon after that for aiming at advanced gastrointestinal cancers by leveraging the established safety profile from phase I.
Next-generation anti-TACA therapy: GNX201 etc
- Pro-antibody
- BsAb
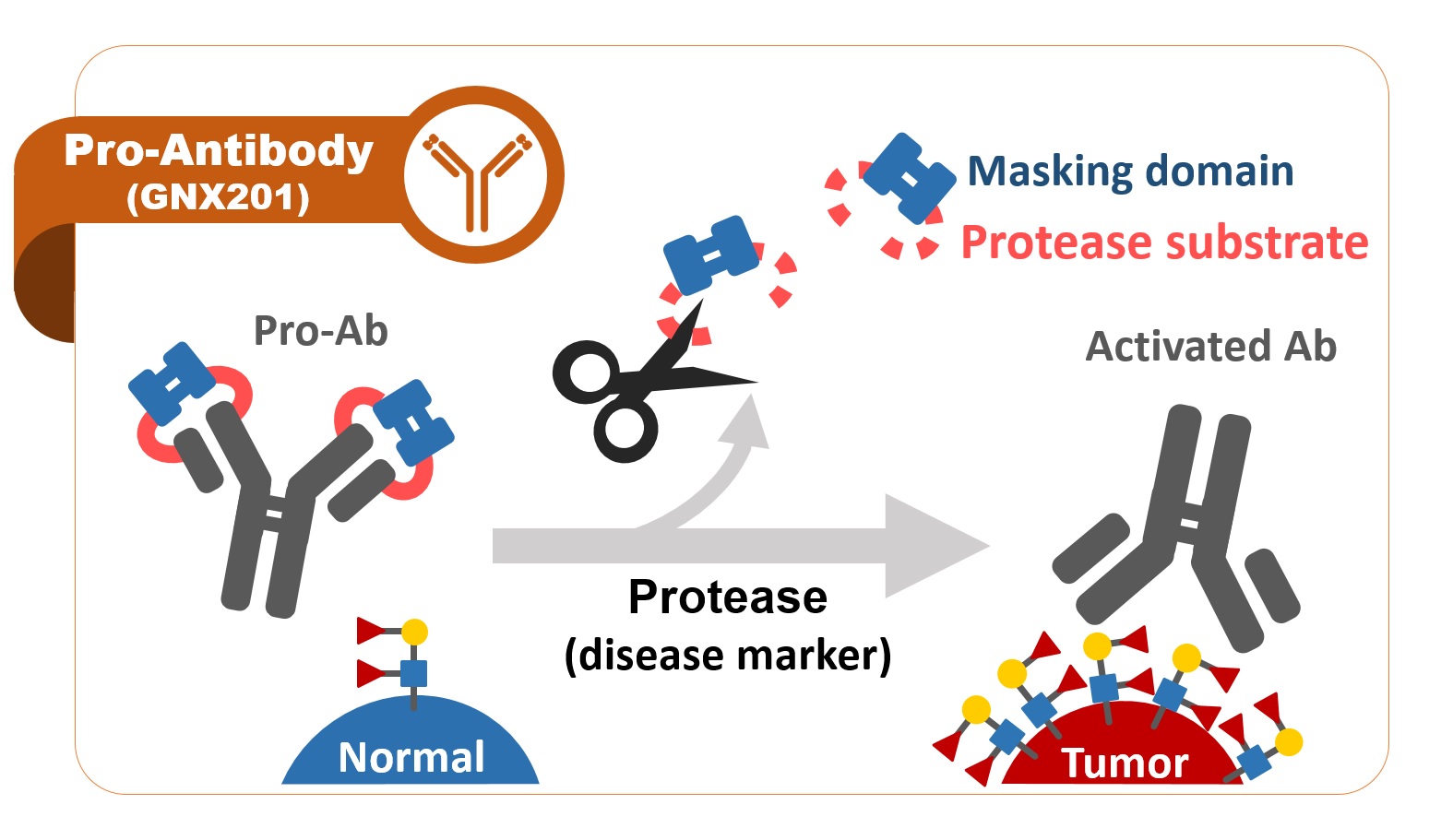
Many cancer-upregulated antigens may have weak-to-moderate expression in tumor-adjacent or -distal normal organs. To reduce off-tumor side effects of current targeted therapy, mAbs with high tumor selectivity are more favorable candidates for their accurate on-tumor targeting. In this regard, GlycoNex has been developing GNX201, an anti-TACA pro-antibody that applies a proprietary “Antibody Lock” technology by fusing a masking peptide to the variable region of the anti-TACA mAb via a cleavable linker. By design the generated pro-antibody is “turned on” by dropping the masking peptide in tumor microenvironment whereas it remains an off state with the masked domain in circulation. The selective activation in tumors allows GNX201 to accurately target tumors rather than doing harm to antigen-present normal organs. In preclinical studies, we have demonstrated that GNX201 has remarkably lower normal tissue reactivity, capability of tumor-localized activation, and comparable tumor growth inhibition in vivo to the unmasked mAb in xenograft models. Those results indicate that GNX201, based on pro-antibody design, has high potential to overcome the safety issues caused by off-tumor effect without compromising the efficacy of mAbs.
Biosimilar development
SPD8: Denosumab biosimilarIn highly competitive biosimilar campaigns, GlycoNex has established a well-integrated platform for biosimilar development including cell line generation, process development, and analytical method development. Since 2010, GlycoNex has been cooperating with Mitsubishi Gas Chemical Company, Inc. on Denosumab biosimilar. To date, the biosimilar SPD8 has demonstrated high similarity to the reference medicinal products. SPD8 program has been also in clinical phase I in Japan
- Denosumab
- an anti-RANK ligand humanized IgG2 antibody first approved in 2010.
- Two types of approved indications with different trade names: Prolia for osteoporosis; Xgeva for tumor bone metastases and giant cell tumor of bone.
- GlycoNex co-develops SPD8 with Mitsubishi Gas Chemical Company and Cultivecs.